BloodSTOP® iX Advanced Hemostat with WoundHEAL®
BloodSTOP® iX Advanced Hemostat with WoundHEAL® is a clinically proven, research-based hemostatic dressing and wound healing matrix that provides rapid hemorrhage control, dramatically reducing blood loss. It is an all-natural, plant-based, biocompatible, water soluble, etherified carboxymethyl cellulose (ECMC) matrix that utilizes patented technology to achieve superior hemostasis and fast wound healing.
Well-established as an ideal hemostatic dressing, BloodSTOP iX has demonstrated in an animal model the ability to modulate endogenous tissue cytokines, as well as to bind to cellular receptors on cell membranes and thereby activating Wnt/Frizzled cellular signaling1. This leads to activation of endogenous stem cells and cytokines, which brings about angiogenesis and tissue regeneration. Additionally, it has shown to decrease inflammatory cytokines, thereby reducing the infection and inflammation risk, and promoting fast wound healing1,2.
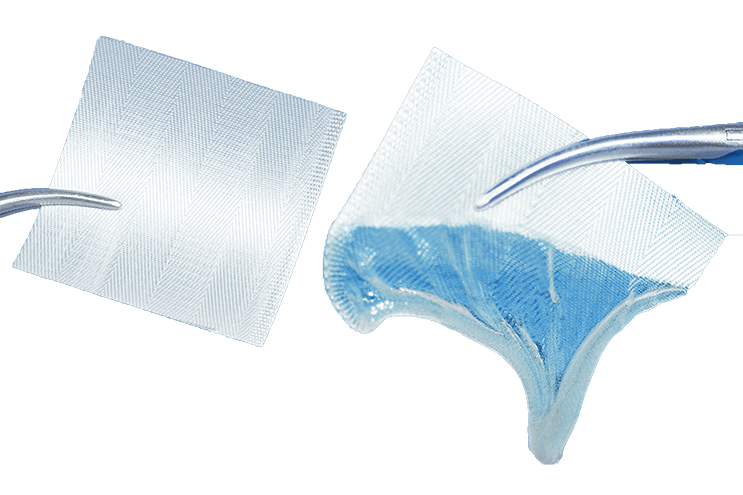
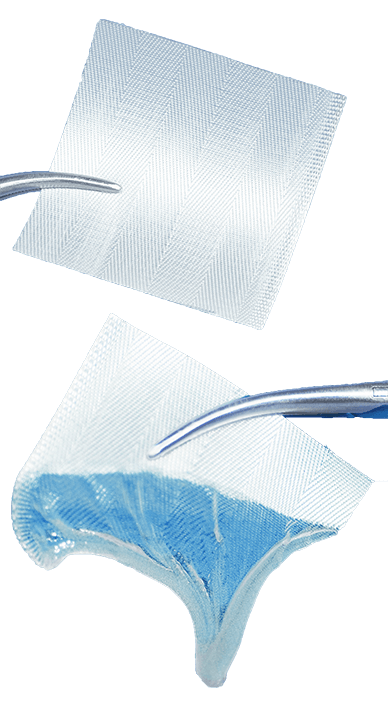
Features |
Benefits |
| Provides rapid hemostasis under a minute3 (from minor to larger wounds). | Dramatically reduces blood loss. |
| Matrix turns into an adhesive gel upon contact with blood and exudates. | Creates a moist wound environment that assists with natural granulation and epithelialization, promotes wound healing and proliferation of tissue growth. |
| The gel sticks, seals, and protects wound without any blood oozing3. | Prevents contamination, reduces the risk of infection and inflammation. |
| The sticky gel provides long-lasting and stable hemostasis. | No rebleeding and adhesion upon removal of secondary dressing coverage4,5,6. |
| Forms translucent gel seal. | Allows easy monitoring of the wound for damage repair without disrupting blood clotting or needing removal. |
| pH neutral: ~7.2, natural plant-based, nontoxic, no irritation, nonexothermic. | Will not irritate the wound, and reduces pain upon application and removal. |
| 100% absorbable, biocompatible, and biodegradable; can be used on patients with blood thinner medications (EU)4,5. | Leaves no residue in the wound; no reported adverse patient events. |
| Stabilizes wound with less materials than typically needed with other products. | Time and cost-effective treatment. |
| Easy to remove with sterile water or saline. | Softens, loosens, and removes slough and necrotic debris from the wound bed. |
| Gel matrix provides flexibility. | Conforms to any wound: small, large, irregular, acute, chronic, surgical, or trauma injury. |
| Sealed in sterile, lightweight, waterproof packaging. | Easy to carry anywhere. |
Watch How BloodSTOP iX Works
BloodSTOP iX Mechanism of Action Video
Specifications
Ingredients: 100% plant-based, purified, water soluble, etherified natural sodium carboxymethyl cellulose
| Product Name | Order # | Size* | Quantity |
| BloodSTOP iX Advanced Hemostat with WoundHEAL | BS-iX-27 | 0.5″ × 2″ (1.3 × 5 cm) | 24 pc/box |
| BloodSTOP iX Advanced Hemostat with WoundHEAL | BS-iX-14 | 2″ × 2″ (5 × 5 cm) | 12 pc/box |
| BloodSTOP iX Advanced Hemostat with WoundHEAL | BS-iX-15 | 2″ × 4″ (5 × 10 cm) | 12 pc/box |
| BloodSTOP iX Advanced Hemostat with WoundHEAL | BS-iX-17 | 4″ × 8″ (10 × 20 cm) | 12 pc/box |
| BloodSTOP iX Advanced Hemostat with WoundHEAL | BS-iX-20 | 2″ × 14″ (5 × 35 cm) | 12 pc/box |
* Two larger sizes are now available for larger wound or extremity arterial hemorrhage control—BloodSTOP iX Trauma Matrix Hemostatic Dressing.
Applications
References
1 Peng D, B. Reed-Maldonado A, Banie L, Wang G, Lin G, F. Lue T. Carboxymethylcellulose Activates Dermal Cells and Adipose-Derived Stem Cells Through Wnt/β-catenin Pathway. J Surg Res (Houst). 2021;04(01). doi:10.26502/jsr.10020117
2 Ju S, Wang K, Qiao L et al. Application of BloodSTOP iX Wound Heal Nanocellulose Matrix for Burn Wound Care. J Surg Res (Houst). 2021;04(01). doi:10.26502/jsr.10020105
3 Ethox International, Rush, NY, Hemostasis Assessment of BloodSTOP, BloodSTOP iX, GLP-2006-0332, 2006.
4 Ferretti L, Qiu X, Villalta J, Lin G. Efficacy of BloodSTOP iX, Surgicel, and Gelfoam in Rat Models of Active Bleeding From Partial Nephrectomy and Aortic Needle Injury. Urology. 2012;80(5):1161.e1-1161.e6. doi:10.1016/j.urology.2012.06.048
5 BloodSTOP iX has European Union CE Class III absorbable implant certification, and for use with anticoagulant medications (blood thinners). BloodSTOP iX currently has US FDA 510(k) clearance for topical wound hemostasis.
6 Li H, Wang L, Alwaal A et al. Comparison of Topical Hemostatic Agents in a Swine Model of Extremity Arterial Hemorrhage: BloodSTOP iX Battle Matrix vs. QuikClot Combat Gauze. Int J Mol Sci. 2016;17(4):545. doi:10.3390/ijms17040545